Rapid response through the simple and quick method diagnosis,
minimizing the economic loss of customers.
LIVESTOCK ANIMALS
RIDX®
Bovine Cryptosporidium Ag Test Kit
[LGM-BCG-11]
DESCRIPTION
Bovine cryptosporidiosis, a gastrointestinal disease of cattle, is caused by the protozoan parasite Cryptosporidium. It's a major cause of neonatal calf diarrhea and can be fatal if not managed properly. The infection constitutes a pervasive global phenomenon that results in substantial economic losses within the beef and dairy industries.
The RIDX® Bovine Cryptosporidium Ag Test Kit is a lateral flow chromatographic immunoassay for the qualitative detection of Cryptosporidium in bovine feces. This kit shows two letters which are the test (T) line and the control (C) line on the surface of the device. If the Cryptosporidium antigen exists in the sample, it binds to the gold-conjugated anti-Cryptosporidium antibody. The antigen-antibody complex moves through the membrane by capillary force and responds to the secondary anti-Cryptosporidium antibody on the test line, resulting in a red line. The control line indicates that the test is performed correctly and should appear when the test is complete.
Two different monoclonal antibodies to the oocyst of Cryptosporidium parvum are used as a capture and detector in the kit. The RIDX® Bovine Cryptosporidium Ag Test Kit can detect Cryptosporidium in bovine feces with high accuracy.
SPECIMEN
Bovine feces
COMPONENTS
• RIDX Cryptosporidium Ag test device (10 tests)
• Sample dilution buffer (10 tubes)
• Disposable swab (10 ea)
• Dropper cap with filter (10 ea)
• Paper rack for standing buffer tubes (1 ea)
• Instructions for use (1 sheet)
FEATURES
• Sensitivity: 95.56% (43/45) vs. PCR
• Specificity: 99.64% (278/279) vs. PCR
• Diagnostic Agreement: 99.07% (321/324) vs. PCR
• Limits of Detection: 102 oocysts/mL
• No cross-reactivity with other bovine pathogens (Aino virus, Akabane virus, BCV, BHV-1, BRSV, Rotavirus, Escherichia coli K99, Giardia lamblia, Parainfluenza virus SF-4)
PACKAGE
• 10 Tests/Kit [LGM-BCG-11]
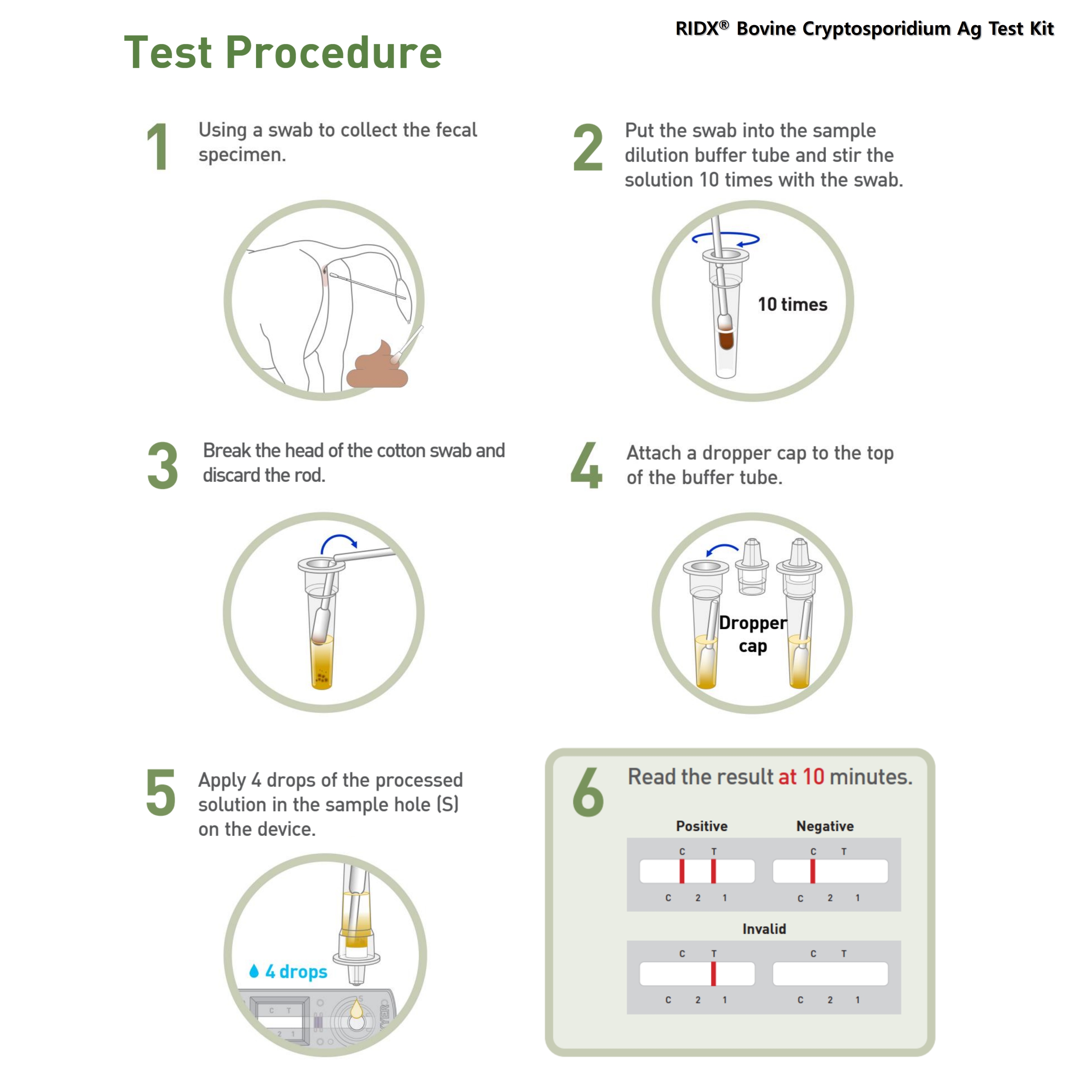
TEST PROCEDURE
1. All samples and test components should be at room temperature (15~30°C/59~86℉) before use.
2. Using a swab to collect fecal specimen.
3. Put the swab into the sample dilution buffer tube and stir the solution 10 times with the swab to disperse the specimen into the buffer.
4. Break the head of the cotton swab and discard the rod.
5. Attach a dropper cap to the top of the buffer tube.
6. Apply 4 drops (approximately 100 μL) of the processed solution in the sample hole on the device.
7. Read test result at 10 minutes.